Introduction
Batteries power many of the devices we use every day — from remote controls and flashlights to smartphones and electric vehicles. Although they come in different shapes and sizes, the basic principle behind how batteries work remains the same.
In this guide, we will explore:
-
What a battery is
-
How batteries generate electricity
-
The difference between AA and lithium-ion batteries
-
Common battery types and their uses
-
Safety and maintenance tips
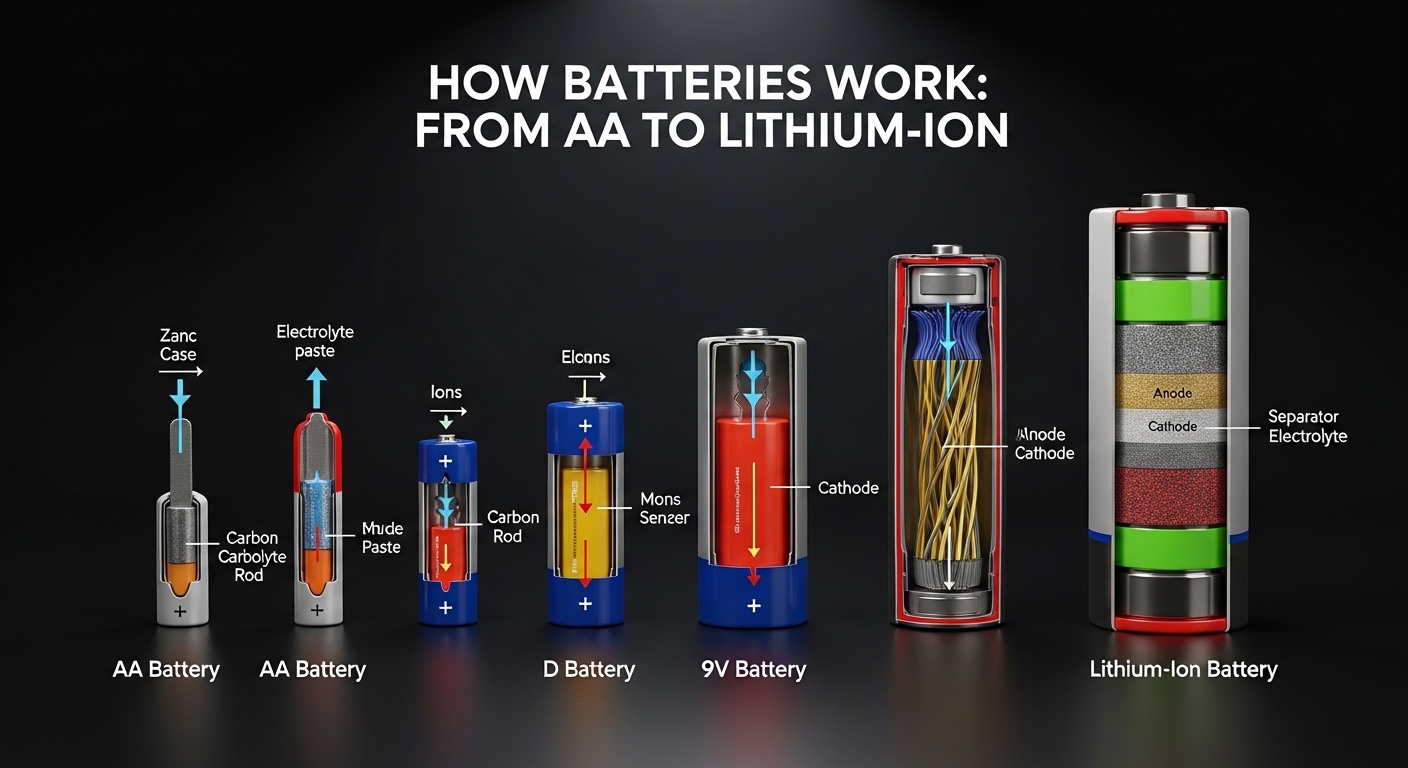
What Is a Battery?
A battery is a device that stores chemical energy and converts it into electrical energy. It provides power to electronic devices without needing a direct connection to the electrical grid.
At its core, a battery contains three main components:
-
Anode (negative electrode)
-
Cathode (positive electrode)
-
Electrolyte (chemical medium that allows ions to move)
When a battery is connected to a device, a chemical reaction occurs inside it. This reaction creates a flow of electrons, which generates electricity.
How Do Batteries Generate Electricity?
The process may sound complex, but the idea is simple.
Here’s how it works step by step:
-
A chemical reaction occurs between the anode and cathode.
-
This reaction releases electrons at the anode.
-
The electrons travel through an external circuit (your device).
-
The electrons return to the cathode.
-
Ions move through the electrolyte to balance the charge.
This continuous movement of electrons creates an electric current that powers your device.
Once the chemical materials inside the battery are used up, the battery either needs to be replaced or recharged, depending on its type.
Understanding AA Batteries
AA batteries are one of the most common battery sizes used worldwide. They are typically used in:
-
Remote controls
-
Wall clocks
-
Flashlights
-
Toys
-
Wireless keyboards
Most standard AA batteries are alkaline batteries, which are single-use (non-rechargeable).
Key Features of AA Alkaline Batteries
-
Nominal voltage: 1.5 volts
-
Designed for moderate power usage
-
Long shelf life
-
Affordable and widely available
Alkaline batteries are reliable for low- to medium-drain devices but are not ideal for high-performance electronics.
What Are Rechargeable Batteries?
Rechargeable batteries can be used multiple times by restoring their chemical energy using an external power source.
Common rechargeable battery types include:
-
Nickel-Metal Hydride (NiMH)
-
Nickel-Cadmium (NiCd)
-
Lithium-ion (Li-ion)
Rechargeable batteries are commonly used in:
-
Cameras
-
Power tools
-
Portable speakers
-
Laptops
-
Smartphones
They reduce long-term waste and can be more economical over time.
How Lithium-Ion Batteries Work
Lithium-ion batteries are widely used in modern electronics because of their high energy density and lightweight design.
Unlike alkaline batteries, lithium-ion batteries:
-
Are rechargeable
-
Store more energy in a smaller size
-
Have a longer lifespan
How Lithium-Ion Technology Works
Inside a lithium-ion battery:
-
Lithium ions move from the anode to the cathode during discharge.
-
When charging, the ions move back to the anode.
-
This movement of lithium ions creates the electric current.
Lithium-ion batteries are commonly found in:
-
Smartphones
-
Tablets
-
Laptops
-
Electric vehicles
-
Power banks
They are efficient and powerful but require built-in safety systems to prevent overheating.
Comparing AA and Lithium-Ion Batteries
Here is a simple comparison:
AA Alkaline Batteries
-
Single-use
-
Lower energy capacity
-
Suitable for low-drain devices
-
Widely available
Lithium-Ion Batteries
-
Rechargeable
-
High energy density
-
Used in high-performance electronics
-
Longer lifespan
The choice depends on the device and how much power it requires.
Primary vs Secondary Batteries
Batteries are generally divided into two main categories:
1. Primary Batteries (Non-Rechargeable)
-
Designed for one-time use
-
Examples: Alkaline, Zinc-Carbon
-
Common in household devices
2. Secondary Batteries (Rechargeable)
-
Can be recharged multiple times
-
Examples: Lithium-ion, NiMH
-
Used in portable electronics
Understanding this difference helps you choose the right battery type for your needs.
Factors That Affect Battery Performance
Battery performance can vary depending on several conditions:
-
Temperature (extreme heat or cold reduces efficiency)
-
Storage conditions
-
Device power demand
-
Charging habits (for rechargeable batteries)
-
Age of the battery
Proper care can significantly extend battery life.
Battery Safety Tips
Batteries are generally safe when used properly. However, it is important to follow basic safety guidelines:
-
Do not mix old and new batteries in the same device.
-
Avoid exposing batteries to high heat.
-
Do not puncture or crush batteries.
-
Use the correct charger for rechargeable batteries.
-
Recycle used batteries according to local regulations.
Lithium-ion batteries, in particular, should be handled carefully to avoid overheating or damage.
Environmental Considerations
Battery disposal is an important environmental issue.
To reduce environmental impact:
-
Use rechargeable batteries when possible.
-
Recycle batteries through approved collection programs.
-
Avoid throwing batteries in household waste.
Many regions have battery recycling centers that safely process used batteries.
Conclusion
Batteries play a vital role in modern technology, powering everything from small household devices to advanced electronics. While AA alkaline batteries are simple and reliable for everyday use, lithium-ion batteries offer higher performance and rechargeability for modern devices.
By understanding how batteries work — including their components, chemical processes, and differences — you can make informed choices about which battery type best suits your needs.
Whether you are using a remote control or a smartphone, the science behind the power source remains both fascinating and practical.
Frequently Asked Questions (FAQ)
1. Why do some batteries last longer than others?
Battery life depends on the type of battery, the device’s power demand, and environmental conditions.
2. Can I replace alkaline batteries with rechargeable ones?
In many cases, yes — but always check the device specifications for voltage compatibility.
3. Why do lithium-ion batteries degrade over time?
Rechargeable batteries gradually lose capacity due to repeated charging cycles and chemical aging.
4. Is it safe to leave devices charging overnight?
Modern devices include protection systems, but prolonged charging over time may contribute to battery wear.
